Study title
“Effect of Synbiotics on the Gut Microbiota of Cesarean Delivered Infants: a Randomized, Double-blind, Multicenter Study.“
Official registration: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2838
Study Objective
To investigate the effect of scGOS/lcFOS and Bifidobacterium breve M-16V on the gut microbiota of caesarean-born infants.
Description
Caesarean birth has been associated with increased risk of immune and metabolic diseases later in life, likely due to the altered gut microbiota. The gut microbiota acts as a potential modifiable risk factor for disease development.
Studies depicted a delayed colonization of keystone microbial colonizers, such as Bifidobacterium in Caesarean-born infants. Because early life is a critical period, as the infant’s immune system is still maturing and is influenced by the gut microbiota, any dysbiosis as a result of environmental factors such as delivery mode could lead to long-lasting health effects
Methodology
The JULIUS SN study1Chua MC, et al. J Pediatr Gastroenterol Nutr. Published on 2017;65:102–6 was a randomised, double-blind, controlled intervention study investigating the effect of our synbiotic blend (scGOS/lcFOS and B. breve M-16V) on the gut microbiota of Caesarean born infants.
153 term infants delivered by C-section were randomly assigned to one of three formula groups from birth until week 16:
- 0g scGOS/lcFOS [Control Group] (n=50)
- 8 g/ 100ml scGOS/lcFOS [Prebiotic Group] (n=51)
- 8 g/ 100ml scGOS/lcFOS and 7.5108 cfu/100 mL B. breve M-16V [Synbiotic Group](n=52)
There was also a reference group of 30 non-randomised, vaginally born infants.
All infants included were mixed-fed; indeed most subjects received the study product corresponding to their allocated group in addition to breastfeeding.
Countries: Singapore, Thailand
Study results
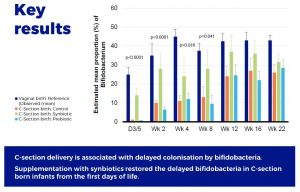
Supplementation with scGOS/lcFOS and Bifidobacterium breve M-16V compensates the delayed Bifidobacterium colonisation in C-section–delivered infants, and modulates the production of acetate and the acidification of the gut.
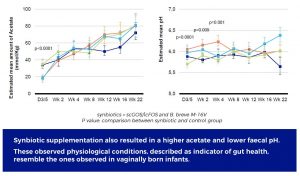
These observed physiological conditions, described as indicator of gut health, emulate the ones observed in vaginally born infants.
The findings confirmed a delayed intestinal colonization by Bifidobacterium species in C-section–delivered infants. This delayed settlement of bifidobacteria was apparent from the first days of life and persisted until 2 to 3 months of age.
However, the early supplementation with scGOS/lcFOS and B. breve M-16V resulted in an immediate colonization by Bifidobacterium, suggesting that the first 3 months of life represent a window of opportunity for a fast recovery of Bifidobacterium colonization in C-section–delivered infants. The relevance of preventing the delayed colonization by Bifidobacterium in C-section born infants lies in the recognized contributory role of Bifidobacterium in early life immune programming. Members of the genus Bifidobacterium have been depicted as microbial biomarker of immune fitness in healthy infants. Supporting the development of the gut microbiota in early life and the typical Bifidobacterium species found in healthy vaginally born and breast-fed infants may improve certain immune phenotypes that are particularly relevant for C-section born infants.
In addition, the study showed that the synbiotic modulation of the gut microbiota resulted in increased acetate. This production of acetate contributed to the acidification of the intestinal milieu. Both physiological parameters have been described as key indicators of gut health.
Download the study summary and key results.
View References
| 1 | Chua MC, et al. J Pediatr Gastroenterol Nutr. Published on 2017;65:102–6 |
|---|